‘Embryo Models’ Challenge Legal, Ethical and Biological Concepts
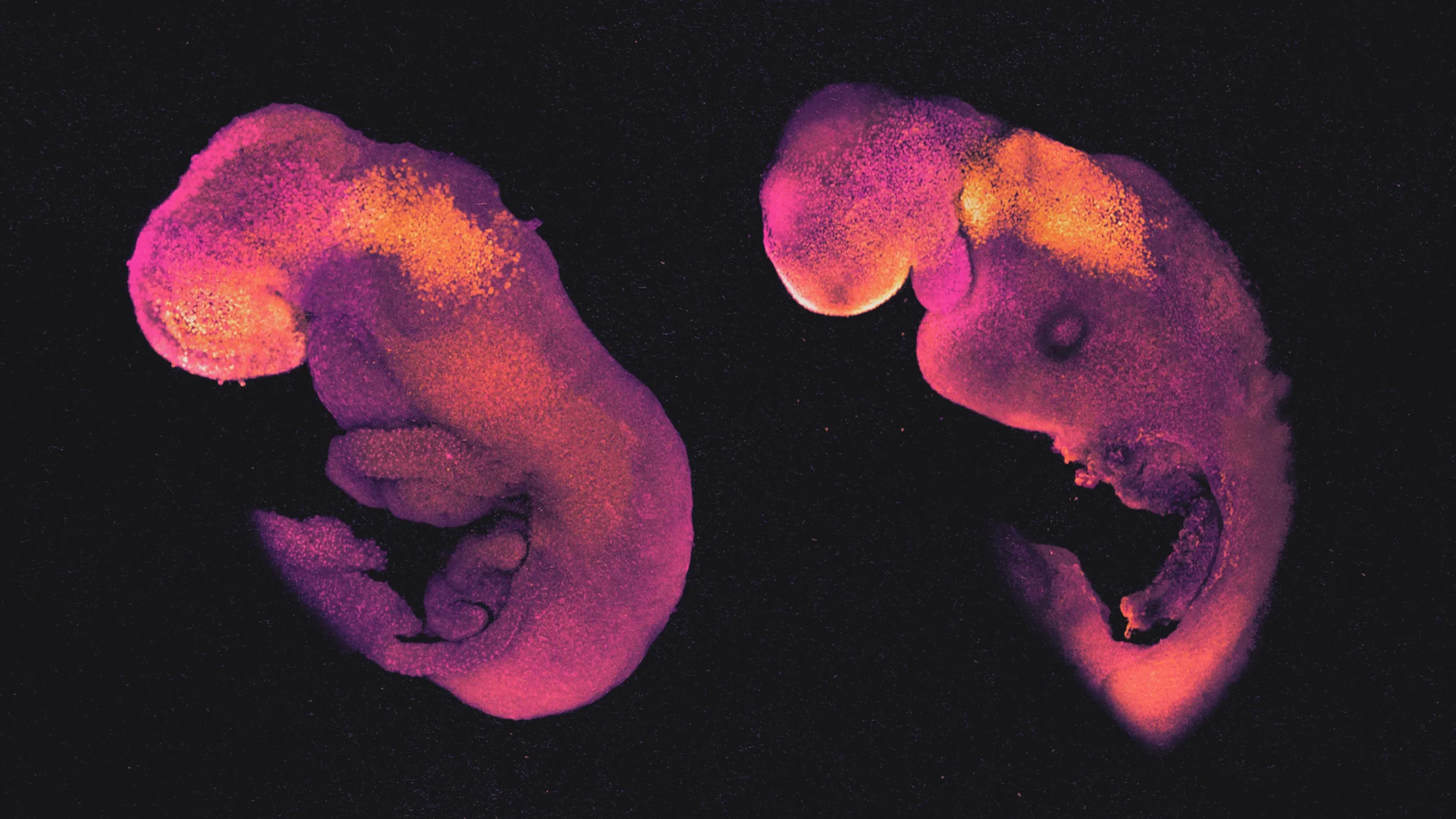
The normal mouse embryo at left is in its eighth day of development and has structures that mark the beginnings of its brain, heart and other organs. At right is a comparably developed synthetic embryo model, grown entirely from mouse stem cells instead of a fertilized egg. Their strong similarity underscores the promise and the concerns about embryo model technology.
University of Cambridge
Introduction
In April, researchers in China reported that they had initiated pregnancies in monkeys through a procedure seemingly much like in vitro fertilization (IVF), in which embryos created in a dish were implanted in the uteruses of cynomolgus monkeys. There seemed nothing remarkable about that — except that this was not genuine IVF, because the embryos had not been produced by fertilization. They had been constructed from scratch from monkey embryonic stem cells, with no egg or sperm involved. They were not real embryos at all, but what many researchers call embryo models (or sometimes “synthetic embryos”).
The multi-institutional team of researchers, led by Zhen Lu (opens a new tab) at the State Key Laboratory of Neuroscience in Shanghai, grew the embryo models (opens a new tab) in vitro to a roughly nine-day stage of development, making them equivalent to what is called a blastocyst in normal embryos. Then they transferred the models into eight female monkeys. In three of the monkeys, the models successfully implanted in the uterus and continued to develop. None of the pregnancies lasted more than a few days, however, before spontaneously terminating.
Meanwhile, other research groups showed last year just how far these embryo models made from stem cells can develop toward whole organisms. Teams led by Magdalena Zernicka-Goetz (opens a new tab) at the University of Cambridge and by Jacob Hanna (opens a new tab) at the Weizmann Institute of Science in Rehovot, Israel, both made them from mouse stem cells and grew them in rotating glass bottles filled with nutrients, which acted like a kind of crude artificial uterus. After about eight days, it was possible to make out the central axis that would, in a normal embryo, become a spinal column, along with the bulbous blob of the nascent head and even a primitive beating heart. You’d need to be an expert to distinguish these living entities from real mouse embryos at a comparable developmental stage.
No one is entirely sure what embryo models are — biologically, ethically or legally — or what they could ultimately become. They could be immensely useful for research, revealing aspects of our developmental processes previously beyond the reach of experiments. They might someday even be used to provide tissues and miniature organs for surgical transplantation. But they also raise profound ethical and philosophical questions.

Magdalena Zernicka-Goetz of the University of Cambridge, a leader in the creation of synthetic embryo models from stem cells, is uncertain about how far their development can be pushed without a good substitute for a placenta.
Simon Zernicki-Glover
Until recently, embryo models bore only a sketchy resemblance to real embryos, and then only at the very earliest stages of growth. But the latest experiments by Zernicka-Goetz, Hanna and others, including the implantation experiments in Shanghai, now force us to wonder how well and how far these entities can reenact the growth of natural embryos. Even if it is currently a distant hypothetical prospect, some researchers see no reason why embryo models might not eventually have the potential to develop all the way into a baby.
There is no clear scientific or medical reason to allow them to do that, and plenty of ethical and legal reasons not to. But even their use as experimental tools raises urgent questions about regulating them. How far should embryo models be allowed to develop before we call a halt to the work? There are currently no clear regulations constraining their creation, nor any consensus on what new regulations should look like. Promising as embryo models are, they raise concerns that the research is running ahead of our ability to decide on its ethical limits.
“Embryo models hold the promise, or threat, of not just creating a realistic model of the development of some parts of important human organs, but of leading to realistic models for all human organs and tissues,” said Hank Greely (opens a new tab), a law professor and chair of the steering committee for the Center for Biomedical Ethics at Stanford University — “and potentially, of creating new babies.”
But beyond ethical concerns, embryo models raise questions about the very definition of personhood and what counts as human. They challenge how we think about what we are.

Hank Greely of Stanford University, an authority on the legal and ethical standing of embryo research, thinks that if human embryo models ever seem capable of producing babies, they will deserve the same ethical and legal protections as real embryos.
Eleanor Greely
Rethinking the 14-Day Rule
Textbooks confidently describe how a fertilized human egg gradually progresses from a uniform ball of cells to a shrimp-shaped implanted embryo to a recognizably human fetus. But we know disturbingly little about that process because some details of it can’t be studied in the womb without compromising the safety of the embryo. And in many countries, it is legal for human embryos to be grown and studied in vitro for only up to 14 days, after which they must be terminated.
That two-week point is when one of the most crucial stages of development occurs, called gastrulation. As the developmental biologist Lewis Wolpert (opens a new tab) put it, “It is not birth, marriage or death, but gastrulation which is truly the most important time in your life.” That is when the rather featureless blob of embryonic cells starts to fold and rearrange itself to acquire the first hints of body structure. The cells begin to specialize into the tissues that will form the nerves, internal organs, gut and more. A central furrow called the primitive streak develops as the precursor to the spinal column, defining the nascent body’s central axis of bilateral symmetry.
In 1990, following reports from the U.S. Department of Health, Education and Welfare and the U.K. Warnock Committee years earlier, many countries decided that the formation of the primitive streak at 14 days should mark the limit for how long human embryos could be sustained in vitro. This 14-day rule was subsequently implemented in the guidelines (opens a new tab) of the International Society for Stem Cell Research (opens a new tab), which are widely followed by scientists worldwide. For decades, it was a comfortable restriction, since human embryos generally stopped growing in vitro after only five to six days, around the stage when they would normally implant in the uterine lining.
In 2016, however, Zernicka-Goetz’s team at Cambridge and the developmental biologist Ali Brivanlou (opens a new tab) at Rockefeller University and his colleagues both showed that they could grow IVF mouse embryos (opens a new tab) all the way up to the gastrulation stage (opens a new tab), using a soft polymer gel matrix as a kind of uterine surrogate.
Furthermore, Hanna and his co-workers showed in 2021 that they could grow natural mouse embryos in vitro far beyond gastrulation (opens a new tab). Using their rotating bioreactor, in which the embryos were sustained in a nutrient solution and an atmosphere with precisely controlled oxygen and carbon dioxide levels, the team grew mouse embryos for 12 days, half of the full gestation period for mice. Hanna thinks the technology could also work with human embryos and could perhaps grow them for many weeks — if the aims of the science justified the project responsibly and the law did not forbid it.
Recognizing the new potential for finding out useful information about how human embryos develop post-gastrulation, the International Society for Stem Cell Research revised its guidelines in 2021. It now recommends that the 14-day limit on human embryo research be relaxed on a case-by-case basis if a good scientific case can be made for extending it. No country has yet modified its laws to take advantage of that latitude.
Embryo models might offer a way to go down that path with even fewer legal and ethical restrictions. They are not legally considered to be embryos because they do not have the potential to grow into viable organisms. So even under present guidelines and regulations in many countries, if embryo models can be grown through gastrulation and beyond, it could become legal for the first time to experimentally study human development and perhaps lead to a better understanding of defects that cause miscarriages or deformities.
But if embryo models can indeed grow that far, at what point do they stop being models and become equivalent to the real thing? The better and further along the models get, the blurrier the biological and ethical boundaries become.
That dilemma was hypothetical when embryo models could only recapitulate the very earliest stages of development. It isn’t anymore.
Turning Stem Cells Into Embryos
Embryo models are generally made from embryonic stem cells, “pluripotent” cells derived from early embryos that can develop into every tissue type in the body. By the time an embryo has reached the blastocyst stage — around day 5 or 6 in human development — it consists of several cell types. Its hollow shell is made of cells that will form the placenta (called trophoblast stem cells, or TSCs) and the yolk sac (the extra-embryonic endoderm, or XEN cells). The pluripotent cells that will become the fetus are confined to a blob on the inside of the blastocyst wall, and it is from them that embryonic stem cells can be cultured.
Experiments in the 1990s and early 2000s showed that embryonic stem cells extracted from one blastocyst and transferred into another can still become an embryo capable of developing all the way to full-term birth as a healthy animal. But the support provided by TSCs and XEN cells is essential — embryonic stem cells alone can’t get past the first few days of development unless they are in a blastocyst.
More recent research, however, shows that embryolike structures can be made from scratch from the respective cell types. In 2018, Zernicka-Goetz and her colleagues showed that assemblies of embryonic stem cells, TSCs and XEN cells from mice could self-organize into a hollow form shaped like a peanut shell and comparable in appearance to a regular embryo undergoing gastrulation. As gastrulation proceeded, some of the embryonic stem cells showed signs of getting more specialized and mobile as a prelude to the development of internal organs.
But those early embryo models were flawed, Zernicka-Goetz said, because the added XEN cells were at too late a developmental stage to wholly fulfill their role. To solve that problem, in 2021 her group found a way to convert embryonic stem cells into early-stage XEN cells. “When we placed [embryonic stem cells], TSCs and these induced-XEN cells together, they could now undergo gastrulation properly (opens a new tab) and initiate development of organs,” she said.
Last summer in Nature, Zernicka-Goetz and her collaborators described how they had used a rotating bottle incubator to extend the growth (opens a new tab) of their mouse embryo models by another crucial 24 hours, to day 8.5. Then the models formed “all regions of the brain, beating hearts and so on,” she said. Their trunk showed segments arising for development into different parts of the body. They had a neural tube, a gut and the progenitors of egg and sperm cells.
In a second paper published around the same time in Cell Stem Cell, her group induced embryonic stem cells to become TSCs (opens a new tab) as well as XEN cells. Those embryo models, cultivated in the rotating incubator, developed to the same advanced stage.
Merrill Sherman/Quanta Magazine; source: 10.1038/s41586-022-05246-3 (opens a new tab)
Meanwhile, Hanna’s team in Israel was growing mouse embryo models in a similar way, as they described in a paper in Cell (opens a new tab) that was published shortly before the paper from Zernicka-Goetz’s group. Hanna’s models too were made solely from embryonic stem cells, some of which had been genetically coaxed to become TSCs and XEN cells. “The entire synthetic organ-filled embryo, including extra-embryonic membranes, can all be generated by starting only with naïve pluripotent stem cells,” Hanna said.
Hanna’s embryo models, like those made by Zernicka-Goetz, passed through all the expected early developmental stages. After 8.5 days, they had a crude body shape, with head, limb buds, a heart and other organs. Their bodies were attached to a pseudo-placenta made of TSCs by a column of cells like an umbilical cord.
“These embryo models recapitulate natural embryogenesis very well,” Zernicka-Goetz said. The main differences may be consequences of the placenta forming improperly, since it cannot contact a uterus. Imperfect signals from the flawed placenta may impair the healthy growth of some embryonic tissue structures.
Without a better substitute for a placenta, “it remains to be seen how much further these structures will develop,” she said. That’s why she thinks the next big challenge will be to take embryo models through a stage of development that normally requires a placenta as an interface for the circulating blood systems of the mother and fetus. No one has yet found a way to do that in vitro, but she says her group is working on it.
Hanna acknowledged that he was surprised by how well the embryo models continued to grow beyond gastrulation. But he added that after working on this for 12 years, “you are excited and surprised at every milestone, but in one or two days you get used to it and take it for granted, and you focus on the next goal.”
Jun Wu (opens a new tab), a stem cell biologist at the University of Texas Southwestern Medical Center in Dallas, was also surprised that embryo models made from embryonic stem cells alone can get so far. “The fact that they can form embryolike structures with clear early organogenesis suggests we can obtain seemingly functional tissues ex utero, purely based on stem cells,” he said.
In a further wrinkle, it turns out that embryo models do not have to be grown from literal embryonic stem cells — that is, stem cells harvested from actual embryos. They can also be grown from mature cells taken from you or me and regressed to a stem cell-like state. The possibility of such a “rejuvenation” of mature cell types was the revolutionary discovery (opens a new tab) of the Japanese biologist Shinya Yamanaka, which won him a share of the 2012 Nobel Prize (opens a new tab) in Physiology or Medicine. Such reprogrammed cells are called induced pluripotent stem cells, and they are made by injecting mature cells (such as skin cells) with a few of the key genes active in embryonic stem cells.
So far, induced pluripotent stem cells seem able to do pretty much anything that real embryonic stem cells can do, including growing into embryolike structures in vitro. And that success seems to sever the last essential connection between embryo models and real embryos: You don’t need an embryo to make them, which puts them largely outside existing regulations.
Growing Organs in the Lab
Even if embryo models have unprecedented similarity to real embryos, they still have many shortcomings. Nicolas Rivron (opens a new tab), a stem cell biologist and embryologist at the Institute of Molecular Biotechnology in Vienna, acknowledges that “embryo models are rudimentary, imperfect, inefficient and lack the capacity of giving rise to a living organism.”
The failure rate for growing embryo models is very high: Fewer than 1% of the initial cell clusters make it very far. Subtle abnormalities, mostly involving disproportionate organ sizes, often snuff them out, Hanna said. Wu believes more work is needed to understand both the similarities to normal embryos and the differences that may explain why mouse embryo models haven’t been able to grow beyond 8.5 days.
Still, Hanna is confident that they will be able to extend that limit by improving the culture device. “We can currently grow [IVF] mouse embryos ex utero until day 13.5 — the equivalent for human embryos will be around day 50 to 60,” he said. “Our system opens the door.”
He added, “When it comes to studying early human development, I believe this is the only possible way.”
Marta Shahbazi (opens a new tab), a cell biologist at Cambridge who works on embryogenesis, agrees. “For humans, an equivalent system [to mouse embryo models] would be really useful, because we don’t have an in vivo alternative to study gastrulation and early organogenesis,” she said.
A human blastocyst six days after fertilization is still just a hollow ball of cells. Until recently, human embryos could not be kept alive in vitro much past this point.
Dr. Yorgos Nikas/Science Source
Already, both Zernicka-Goetz and Hanna are making rapid progress with human embryo models. On June 15, their two groups simultaneously posted (opens a new tab) preprints (opens a new tab) describing the growth of such structures derived entirely from human pluripotent stem cells that they claimed to develop in vitro all the way to a stage equivalent to that of a normal embryo 13 to 14 days after fertilization. The researchers say that their human embryo models show some of the key developmental features of natural ones at this same stage, although those claims have yet to be peer-reviewed. At this rate of advance, it will surely soon be possible in principle to grow these entities beyond the widely observed 14-day legal limit — forcing the question of whether we should.
In theory, human embryo models grown to an advanced stage of development could become sources of organs for transplants and research. “Although the synthetic embryoids we make are distinguishable from natural embryos,” Hanna said, “they still have all [nascent] organs, and in the right position.”
Embryonic and induced pluripotent stem cells in vitro can currently be guided to grow into rudimentary miniature organs (or “organoids”) of pancreatic, kidney and even brain tissue. But organoids typically fail to reproduce the structure of real organs accurately, probably because they lack essential signals and multicellular components that would arise naturally in real embryos. “We anticipate that these defects might be corrected by generating structures that recapitulate natural processes occurring in development,” Zernicka-Goetz said.
Hanna thinks that embryo models could also be used to identify drug targets and screen for novel therapeutics, particularly for reproductive problems such as infertility, pregnancy loss, endometriosis and preeclampsia. “This is providing an ethical and technical alternative to the use of embryos, oocytes or abortion-derived materials and is consistent with the latest ISSCR guidelines,” he said. He has already founded a company to test potential clinical applications of human embryo models.
But Alfonso Martinez Arias (opens a new tab), a developmental biologist at Cambridge and Pompeu Fabra University in Barcelona who studies the role of embryonic stem cells in mammalian development, stresses that such applications remain unproven. He thinks it is hard to see how much could be understood about questions of real embryo growth from the development of such a distorted version.
Besides, he said, none of this has yet been shown in humans. “I do not think we should advance a field through wishful thinking, but with facts,” he said.
The Ethical Frontier
As long as embryo models remain just models, their use in research and medicine may not arouse much controversy. “A basic ethics principle called subsidiarity stipulates that a scientific or biomedical goal should be achieved using the least morally problematic way,” Rivron said. For research on global health concerns such as family planning, he said, studies of embryo models seem like a less ethically challenging alternative than work on IVF embryos.
“We should remember that synthetic embryos are not real embryos,” Hanna said. So far, they lack the crucial potential to grow into a true fetus, let alone a baby: If they are implanted in mice, they don’t develop further.
But the capacity for further development is central to the ethical status of the embryo models, and there’s no guarantee that their current inability to yield fetuses and live births will persist.
Rivron agrees that the work on embryo models that he and others are doing could lead to a new reproductive technology. “We can foresee that the most complete embryo models will at some point tip over to become embryos giving rise to individuals,” he said. “I believe these individuals should be fully entitled as beings, independent of the way they formed.”
For that reason, he is working with ethicists to shape an ethical framework for these studies. “Attempting to use human embryos formed from stem cells for assisted reproduction might become possible one day,” he said, “but it would require an exhaustive prior discussion and evaluation on whether it is safe, socially and ethically justifiable, and desirable.”
But the ethical issues don’t kick in only if the technology is used for human reproduction. Greely believes that “if an embryo model is ‘similar enough’ to a ‘normal’ human embryo, it should be treated as a human embryo for statutory and regulatory purposes, including, but not limited to, the 14-day rule or any revision of it.”
What counts as similar enough? That criterion would be met, he said, “if the embryo model has a significant probability of being able to produce a living human baby.”
The trouble is, it could be very hard to know for sure whether that’s the case short of implanting a human embryo model in a uterus. The only way to determine the ethical status of such an entity might then be unethical.
Work like that of the Chinese team with monkey embryo models, however, might foreclose that uncertainty. If these embryolike entities can induce pregnancies and someday yield offspring in monkeys, we might reasonably infer that equivalent human embryo models could too. In a commentary (opens a new tab) on that work, Insoo Hyun (opens a new tab), the director of research ethics at Harvard Medical School’s Center for Bioethics, wrote: “It is at this point that human embryo models could be deemed to be so accurate that they would amount to being the real thing functionally.”
Such a result, even if only in monkeys, might lead regulators to decide that human embryo models deserve to be treated like embryos, with all the attendant restrictions. Some researchers feel that we urgently need a new definition of an embryo to offer clarity and keep pace with the scientific advances. If there is good reason to suppose an embryo model has the potential to generate viable offspring, we will need to either accept the regulatory implications or find ways to nullify that potential.
These are the dilemmas of a technique that could blur our old ideas about what qualifies as human, and about how people are created. Bartha Maria Knoppers (opens a new tab), a professor and research chair at McGill University in Canada and an authority on research ethics, wrote a commentary (opens a new tab) for Science with Greely in which they described developments like embryo models as “nibbling at the legal definition of what a human is.” The more we discover about how we are made and how we could be, the less clear it is that science can bring clarity to that question.
Editor’s note: This article was updated on June 15, 2023, to include work announced by Zernicka-Goetz and Hanna at press time.
Correction: June 13, 2020
Mention of Zernicka-Goetz’s 2016 paper on growing IVF mouse embryos in a gel matrix was added alongside the citation of Brivanlou’s similar work.
Correction: June 15, 2023
An earlier version of this article incorrectly identified Rivron as a collaborator on research with Zernicka-Goetz. He and many other scientists were coauthors with her on a comment (opens a new tab) in Nature about the ethics of embryo models.